What is ozone?
Ozone gas is a trace gas in the atmosphere. At room temperature and pressure, ozone is colorless at lower concentrations. At concentrations of up to 15% by volume it appears light blue and has an irritating odor. At higher concentrations, the odor of ozone is similar to that of chlorine.
Ozone gas in the environment comes from both natural and man-made sources and is strongly oxidizing. The chemical symbol for ozone is not very different from that of oxygen, which sustains human life, though, and both are composed of the same single element. But the two are the difference between graphite and diamonds: one is heavenly and the other is subterranean.
Why is ozone good in the stratosphere but bad at ground level?
Whether ozone gas is good or bad depends on where it is located. If ozone gas is in the stratosphere, it filters ultraviolet light from sunlight, which is protective of the Earth’s environment. According to the Ozone Depletion Scientific Assessment Report 2018, published on the World Meteorological Organization’s website, for every 1% reduction in stratospheric ozone concentration, UV radiation increases by 2% and skin cancer risk rises by 3%. However, within an altitude range of 1 kilometer above the ground, high concentrations of ozone can cause air quality pollution.
Ozone gas becomes a “health killer” once it reaches the near-surface layer, which is about 10-100 meters above the ground. According to the World Health Organization (WHO), the short-term exposure standard for ozone is 180 micrograms per cubic meter per hour (about 90 ppb). The long-term exposure standard is less than 100 micrograms per cubic meter (about 50 ppb) for 8 hours per year. Near-surface ozone gas hazards are:
- Ozone can stimulate mucous membranes, it is toxic to humans, long time in the air containing 0.1 ppm ozone is not safe to breathe.
- It strongly stimulates the human respiratory tract, causing sore throat, chest tightness and coughing, causing bronchitis and emphysema.
- Ozone can cause neurotoxicity, dizziness, headache, vision loss, memory loss, shortness of breath, fatigue, nose bleeding.
- Ozone can destroy vitamin E in human skin, resulting in wrinkles and dark spots.
- Ozone destroys the body’s immune function, induces chromosomal changes in lymphocytes, accelerates aging, and causes pregnant women to give birth to deformed children.
- Ozone reduces the concentration of chlorophyll, rhodophylls and carbohydrates in plants, which affects photosynthesis and reduces crop yields.
So where does near ground level ozone gas come from?
Near ground level ozone gas, typically is not emitted directly into the air as a pollutant, but rather is formed through a complex photochemical reaction of air pollutants emitted by humans. For example, our industrial production, the driving of oil vehicles, and power plants that use fuel, these activities emit large amounts of nitrogen oxides (NOx) and volatile organic compounds (VOCs), which are converted into ozone by further activities. Therefore, near surface ozone is mainly caused by human activities, and we have unshirkable responsibility for the formation of ozone pollution.
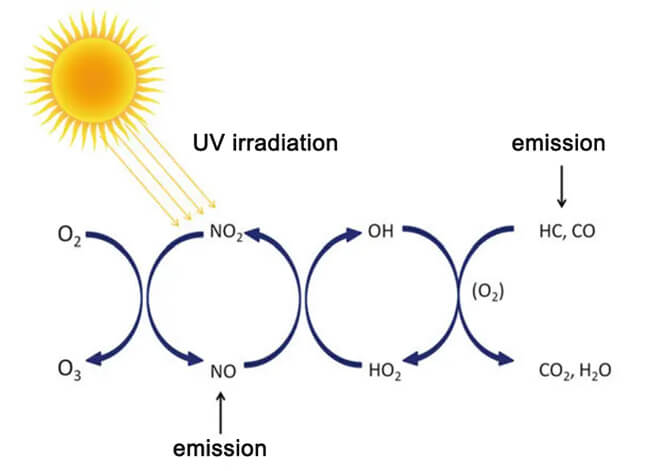
The mechanism of ozone formation due to human activities is very complex, but in simple terms: hydrocarbons and nitrogen oxides (NOx) form ozone in the presence of UV light. In many cases, ozone gas shows a snowballing upward trend. Due to the involvement of volatile organic compounds (VOCs), their free radicals create a chain amplification, resulting in a continuous increase and accumulation of ozone gas. When the sun goes down in the west or other influences, the chain reaction is interrupted and the ozone concentration drops.
What is ozone gas used for?
For the environment
Ozone protects the atmosphere. Ozone gas forms the ozone layer in the Earth’s atmosphere, a layer that effectively absorbs and filters out high-energy ultraviolet (UV) radiation and protects life on Earth from UV damage. In addition, ozone gas is an important oxidizer in the atmosphere. It participates in many chemical reactions, for example, with substances such as nitrous oxide and sulphur dioxide, thereby affecting the redox balance in the atmosphere and regulating the chemical composition of the atmosphere.
For water treatment
Ozone can kill 99% of bacteria and viruses (such as E. coli and the new coronavirus) without any chemical residue. For example, bottled water uses an oxygen-source ozone generator, which has a sterilization efficiency 8-10 times higher than that of an air source and avoids nitrogen affecting the taste of the water. In addition, industrial wastewater contains a large amount of organic matter and dyes that are difficult to decompose, which has a great impact on water bodies and the ecological environment. The strong oxidizing ability of ozone can decompose these difficult-to-decompose organic matter and dyes into harmless substances, thereby achieving the purpose of purifying water quality.
For medical treatment
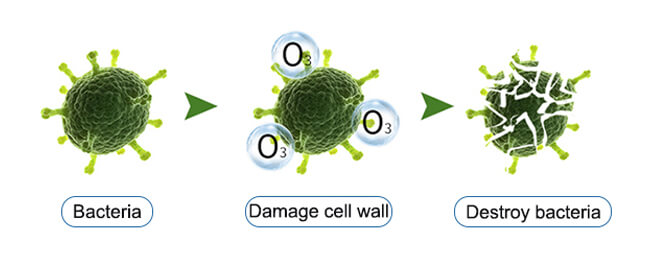
Ozone gas is also important in the medical field and has the unique ability to treat a wide range of diseases. Ozone is capable of oxidizing fungi, bacteria and viruses, destroying their cell walls and cell membrane structures, thus achieving sterilization and disinfection. In addition, a reasonable concentration of ozone can activate cell and tissue activity within the body and improve the body’s resistance. It can promote the normal cellular metabolism, accelerate the healing of wounds and improve the immune function of the body.
For food processing
Ozone also plays an important role in the food processing and storage industry. During food processing, ozone can effectively kill bacteria, mold and other microorganisms to prevent food spoilage and contamination. Ozone treatment can extend the shelf life of fruits and vegetables by 30%-50%. For example, the hardness retention rate of melons can be increased by 20% after refrigeration. In the process of transportation, ozone has strong killing and inhibiting ability for mold, and at the same time, it can absorb the ethylene gas discharged from the respiration of fruits and vegetables, and inhibit the ripening of fruits and vegetables in storage. For food, fruits and vegetables have a better mold, freshness effect.
How can ozone gas levels be measured?
1. Ozone Sensors
Detection is carried out using an ozone sensor. Based on the electrochemical principle, ozone gas concentration is measured by generating a current signal through a chemical reaction between the electrodes on the surface of the sensor and ozone. There are many types of gas sensors for different measurement locations. Common ozone sensors are wall-mounted, duct-mounted and portable.

Wall-mounted ozone sensor
Suitable for monitoring ozone gas in factories, warehouses and other fixed locations.

Duct type ozone sensor
Suitable for monitoring ozone gas in pipelines or confined spaces.

Portable ozone detector
Suitable for professional gas monitors to monitor different places.
2. Ultraviolet light absorption
Measurements are made using the absorption properties of ozone for ultraviolet light. A source of UV light is radiated into a sample of ozone gas, and the difference in intensity between the incident and transmitted light is measured to calculate the ozone concentration.
3. Gas Chromatography
A gas chromatograph is used to separate and measure ozone in a gas sample. Gas chromatography is a more accurate measurement method, but requires specialized instrumentation.
How to choose ozone gas sensor?
When purchasing an ozone gas detector, you must first clarify the characteristics of the use environment and the purpose of use and combine the stability, sensitivity, selectivity, and corrosion resistance of the ozone monitoring equipment to choose.
Stability refers to the stability of the basic response of the sensor during the entire working time, which depends on the zero drift and interval drift. Zero drift refers to the change in sensor output response during the entire working time when there is no ozone. Interval drift refers to the output response change of the sensor continuously placed in ozone, which is manifested as the decrease of the sensor output signal during the working time. Ideally, a sensor under continuous working conditions, the annual zero drift is less than 10%.
Sensitivity refers to the ratio of the ozone gas sensor output change to the measured input change, and it mainly depends on the technology used in the sensor structure. The design principles of most gas sensors use biochemistry, electrochemistry, physics, and optics. The first thing to consider is to select a sensitive technology that has sufficient sensitivity for the detection of the target gas’s valve limit (TLV-threshold limit value) or the lowest explosive limit (LEL-lower explosive limit) percentage.
Selectivity is also called cross-sensitivity. It can be determined by measuring the sensor response produced by a certain concentration of interfering gas. This response is equivalent to the sensor response produced by a certain concentration of ozone gas. This characteristic is very important in the application of tracking multiple gases because the cross-sensitivity will reduce the repeatability and reliability of the measurement. The ideal ozone sensor should have high sensitivity and high selectivity.
Corrosion resistance refers to the ability of the ozone gas sensor to be exposed to a high volume fraction of target gas. When a large number of gas leaks, the probe should be able to withstand 10-20 times the expected gas volume fraction. Under normal working conditions, the sensor drift and zero-point correction value should be as small as possible.