What is evaporation?
Water exists in three states in nature: liquid, gas, and solid. These states can transform into one another. At normal temperatures, water gradually turns into water vapor and disperses into the air, this is evaporation. It can occur at any temperature. For example, water in a bucket dries up under the sun, puddles on the ground slowly disappear, and wet clothes become dry—all are examples of evaporation.
Why evaporation can happen any temperature?
Evaporation is the process where high-energy molecules in a liquid break free from the surrounding molecules and become water vapor. Regardless of the temperature, liquid molecules move randomly at high speeds. Temperature reflects the average energy of all molecules, but some always have higher energy, allowing them to turn into water vapor. Even at very low temperatures, some high-energy molecules can gain enough kinetic energy to overcome surface tension and escape the liquid surface.
According to the Boltzmann distribution, fewer molecules evaporate at lower temperatures, but the number is never zero. This means evaporation can happen at any temperature. However, at higher temperatures, more molecules have sufficient energy to evaporate, making the process faster. At lower temperatures, evaporation happens more slowly.
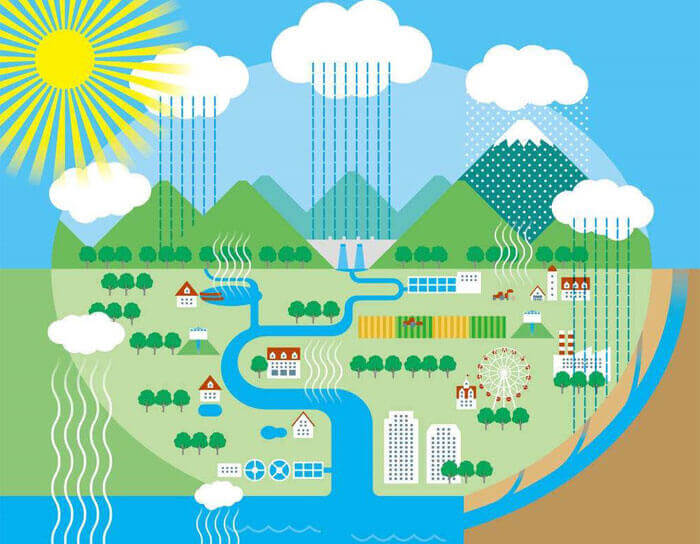
What are the factors that affect evaporation?
The most important factors are temperature, humidity, wind speed, and air pressure.
How does temperature affect water evaporation?
The higher the temperature, the faster water evaporates in nature. This is because increasing the temperature raises the average kinetic energy of liquid molecules, allowing more molecules to break free from the surface and become water vapor in the air. Imagine a pot of water slowly heating on a stove; you’ll see more and more steam rising from the surface as the temperature increases, which is water evaporating at a faster rate. The higher the temperature, the more vigorous the molecular movement, allowing molecules to escape the surface more quickly, naturally leading to faster evaporation.
How does humidity affect water evaporation?
When the air has a high level of water vapor saturation (high humidity), it means the air is already holding a large amount of water vapor and is close to saturation. Under these conditions, the process of water molecules transitioning from liquid to vapor is inhibited because the air is almost unable to accommodate additional water molecules. Furthermore, high humidity increases the water vapor pressure in the air, which further slows down the evaporation rate.
How does wind speed affect water evaporation?
When wind comes into contact with the liquid surface, it carries away the moisture above the surface. This moisture is primarily composed of water molecules that have already evaporated. As the moisture is removed, the air above the liquid surface becomes drier, which means the partial pressure of water vapor in the air decreases.
According to the concept of vapor pressure, when the partial pressure of water vapor in the air above the liquid surface drops below the vapor pressure of the liquid, the liquid will continue to evaporate until a state of equilibrium is finally reached. Therefore, the stronger the wind, the faster the moisture is removed, the drier the air above the surface becomes, and the faster the evaporation rate. Increasing wind speed promotes the diffusion of high-humidity air near the surface, reduces the vapor pressure at the surface.
How does air pressure affect water evaporation?
Air pressure is an important indicator of the atmosphere’s thickness. When the air pressure is high, it means the air density is greater, and the diffusion rate of water molecules from the water surface to the air is slower, which naturally slows down the evaporation rate. When the air pressure is low, it means the air density is relatively lower, allowing water molecules to diffuse more easily into the air, which increases the evaporation rate. Low atmospheric pressure means there are fewer water vapor molecules in the same space, making it easier for water molecules at the surface to escape. This is similar to an empty bus, where passengers waiting for the bus can easily get on, but when the bus is full, the waiting passengers cannot board.
Evaporation video from @ThreeTwentysix
Evaporation vs Volatilization
Differences in properties: Evaporation is the process where molecules at the surface of a liquid escape freely, transitioning from liquid to vapor due to the random motion of water molecules inside the liquid. Volatility refers to the free release and movement of substance molecules, which can occur in both liquids and solids. In addition to, volatility is not affected by temperature.
Differences in occurrence conditions: Evaporation can occur at any temperature, and the higher the temperature, the evaporate faster. Volatility primarily occurs in specific substances like organic compounds, and its occurrence is not influenced by temperature.
Characteristics and influencing factors: Evaporation is a vaporization phenomenon that happens at the surface of a liquid, and it only occurs at the surface, typically at a slower rate. During evaporation, heat is absorbed, which lowers the temperature of the liquid and any objects it is attached to, so it has a cooling effect. Factors influencing evaporate include the liquid’s temperature, surface area, and the airspeed above the liquid’s surface. Volatility is the process where a liquid turns into a gas and disperses into the surroundings at room temperature. Substances like ether, alcohol, and petroleum are volatile. Volatility usually takes longer.
How does evaporation affect weather?
In nature, it can affect weather by affecting energy exchange and water cycle systems.
Impact on energy exchange
When water changes from liquid to vapor, it absorbs a large amount of heat (known as latent heat). The latent heat is quite significant, about 2260 KJ/kg. The amount of heat consumed to evaporate 1 kg of water is equivalent to the amount of heat consumed to heat 538 kg of liquid water to raise its temperature by 1°C. Therefore, during evaporation, water absorbs a substantial amount of heat from the surrounding environment, which leads to a decrease in the temperature of the nearby surroundings.
Impact on water cycle
Evaporation is an important part of the water cycle system. Under the action of gravity, water flows from the mountains to the plains, and then from the plains to the ocean. Evaporation goes against gravity and turns the water in the ocean (the ocean occupies more than 70% of the earth’s surface area, and the evaporated water is mainly the water in the ocean) into water vapor. Under the action of wind, water vapor is carried to the land and mountains. After meeting with cold and hot air, it will form snow and rain. Water vapor falls on the mountains and plains in the form of snow and rain. Therefore, the process of water cycle will affect climate change.
How to measure evaporation outdoors?
1. Manual calculation
Using an evaporating dish is a common manual measurement method. It consists of a moderately deep, wide-mouthed container and a measuring device. To use it, place the container in the area where you want to measure, and add a specific amount of water. Then, place the measuring device on the water surface to record the depth it sinks or the distance the marker moves. Finally, the evaporation rate is calculated using a formula based on the data.
The measuring container should have a smooth surface, be corrosion-resistant, and have uniform thickness, so as not to affect the surface tension of the evaporating water. Since the principle of the evaporating dish is to measure the evaporation amount by evaporating water, the external conditions should be controlled during use to avoid a large amount of water outside the evaporating dish, which will affect the accuracy of the measurement.
2. Evaporation sensor
The evaporation sensor is a commonly used measuring device in outdoor weather stations. It works on the principle of pressure measurement, which calculates the evaporation of water by detecting the weight change of the liquid in the container. This sensor can not only automatically monitor data, but also adapt to various outdoor environments and is not affected by liquid freezing. It can be used with a rain snow sensor, rain gauge, etc. to realize automatic rainfall monitoring.
This sensor usually consists of a sensing element and a signal processor. The sensing element is responsible for detecting changes in physical or chemical properties, such as temperature, pressure, humidity, etc., while the signal processor converts these changes into recognizable electrical signals. This sensor has extremely high measurement accuracy and can accurately capture tiny changes in the evaporation process. It can maintain stable performance even in harsh working environments to ensure data reliability.
3. Reverse osmosis membrane
Reverse osmosis membrane is a method of measurement based on the principle of osmotic pressure. This method requires a transparent film to be laid on the water surface, and water molecules can pass through the film and drift to the outside. The weight change and pressure change of the film are detected in various ways to calculate the value. The characteristic of this membrane is that water molecules can pass through freely, but salt or other impurities cannot pass through. When water molecules pass through the membrane, an osmotic pressure is formed. By measuring this pressure, the evaporation amount can be indirectly calculated.
4. Turbine flowmeter
The turbine flowmeter method uses a rotating sensor to measure the water flow rate and then converts it into evaporation. This method is more complex than the above methods and involves the cooperation between multiple instruments. This method requires precise adjustment and calibration to ensure the accuracy and repeatability of the data.
Conclusion
Nature, with its unique charm, reveals the mysteries of the life cycle, and one essential part of this cycle is evaporate water. Though it may seem like a silent process, it has a profound impact on our environment. Imagine a world without evaporation—water vapor in the sky wouldn’t transform into rain, rivers would cease to flow, and the growth of plants and the survival of animals would be severely affected.
Evaporating water acts as a bridge between the Earth’s surface and the sky, influencing the movement of rivers and shaping the planet’s ecosystems. From agricultural irrigation to large-scale water resource management, such as water diversion projects and the South-to-North Water Transfer Project, every step is accompanied by changes in evaporation. Understanding and mastering these changes scientifically is crucial for human survival and development. Studying evaporate water has significant theoretical value and practical guidance for environmental protection, afforestation, and even land-use strategies.