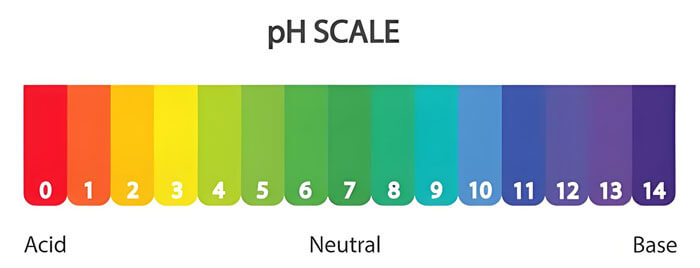
What do pH Mean in Water?
The water pH is an important indicator of its acidity or alkalinity. pH (Potential of Hydrogen) ranges from 0 to 14, with a value of 7 representing neutrality. When the pH is below 7, the water is acidic (the lower the value, the stronger the acidity); when it is above 7, the water is alkaline (the higher the value, the stronger the alkalinity). The World Health Organization (WHO) and the U.S. Environmental Protection Agency (EPA) recommend a drinking water pH range of 6.5 to 8.5. In nature, the ideal pH of water typically ranges from 6.0 to 9.0, while the recommended pH for agricultural irrigation water is usually between 5.5 and 7.5.
What Changes the pH of Water?
Natural Factors
Natural factors are one of the main influences on water pH. The pH of water is affected by atmospheric conditions such as temperature, atmospheric pressure, and rainfall. Water is a powerful solvent and dissolves some of the gases and solids it comes into contact with. For example, carbon dioxide (CO₂) in the air dissolves in water to form carbonic acid, which lowers the pH. Similarly, gases released from volcanic eruptions or wildfires, such as sulfur dioxide (SO₂) and nitrogen oxides (NOₓ), can lead to acidification, especially when they contribute to acid rain.
Geological Factors
Geology also affects water pH. In limestone regions, water tends to have a higher pH (pH 8-9) because limestone mainly consists of calcium carbonate, which dissolves in rainwater to form bicarbonate and hydroxide ions. In volcanic rock regions, the pH of water is lower (pH 5-6) due to the oxidation of sulfides, which forms sulfuric acid and leads to acidic water. The pH of groundwater is also influenced by geological formations and rock types, as different geological conditions result in varying pH levels in groundwater.
Biological Factors
The growth and reproduction of aquatic organisms can cause fluctuations in water pH. When phytoplankton multiply in large numbers, the water pH can rise significantly (sometimes exceeding pH 10). This happens because phytoplankton consume carbon dioxide during photosynthesis, and if the water’s hardness is too low, the pH can spike dramatically. This effect is especially noticeable between 16:00 and 17:00 during summer. On the other hand, aquatic animals contribute to carbon dioxide levels through respiration, which lowers pH. Additionally, the decomposition of organic matter produces acids, further influencing pH levels.
Water Hardness
One of the most crucial factors affecting water pH changes is the balance between dissolved carbon dioxide and carbonate compounds. When carbon dioxide is consumed, if the water lacks sufficient hardness, the pH will rise. Water hardness helps buffer pH fluctuations, which is why maintaining an appropriate hardness level is essential in aquaculture.
Human Factors
Pollutants and waste from human activities can directly or indirectly impact water pH. Industrial, mining, and agricultural discharges introduce various chemicals, including strong acids and bases, altering the pH. For example, fertilizers often contain ammonium compounds, which can lead to pH changes.
Household wastewater typically contains a mix of organic and inorganic substances, which can either raise or lower pH levels. For instance, phosphates and sodium hydroxide in detergents can increase wastewater pH (e.g., laundry wastewater often has a pH of 9-10). Urban runoff can also carry pollutants that disrupt the natural pH balance of water.
How Does pH Affect Water Quality?
Drinking Water Safety
The pH level is a key parameter in determining whether drinking water is safe and healthy. A pH that is too low or too high indicates that the water does not meet quality standards. During the drinking water purification process, coagulants and chlorine-based disinfectants are often added to adjust the pH level. If the pH is too low, it can corrode water distribution pipes, affecting water quality; if it is too high, dissolved salts may precipitate, forming scale deposits. To ensure both safety and taste, the European Union and the World Health Organization (WHO) consistently recommend a drinking water pH range of 6.5 to 8.5 to prevent pipe corrosion while maintaining water quality.
Soil Health
Water quality directly impacts soil’s physical and chemical properties. Chemical components in water, such as minerals, heavy metals, and pH levels, can enter the soil through irrigation and react with existing soil compounds. These reactions may alter soil pH, affecting nutrient availability and microbial activity. For instance, irrigating with acidic water can lead to soil acidification, which may release toxic elements like aluminum and manganese, harming crop growth. Conversely, alkaline water can cause soil alkalization, leading to the precipitation and immobilization of essential micronutrients like iron and zinc, reducing their availability for plants.
Swimming Pool Maintenance
According to guidelines from the WHO and various national health agencies, the recommended pH range for swimming pool water is between 7.2 and 7.8. This range ensures that chlorine-based disinfectants function optimally, effectively controlling bacteria and viruses. Additionally, maintaining the proper pH minimizes eye and skin irritation for swimmers while reducing the risk of corrosion to pool infrastructure, including metal and plastic components.
Aquaculture Improvement
In aquaculture, an excessively high or low pH can negatively affect fish growth, hinder the reproduction of plankton, and increase the occurrence of fish diseases caused by protozoa. When the pH is between 5 and 6.5, dinoflagellates tend to proliferate excessively; when the pH exceeds 8.5, blue-green algae (cyanobacteria) can grow rapidly, deteriorating water quality. The optimal water pH range for aquaculture is generally between 6.5 and 8.5, as this range provides the best conditions for most aquatic organisms to grow and reproduce. However, the ideal pH level may vary depending on the species being cultivated, their growth stages, and the specific aquaculture environment.
Environmental Protection
pH is one of the key indicators monitored by governments and international organizations when establishing environmental protection and water quality standards. Regular monitoring of water pH helps detect abnormalities in water quality early, allowing for timely interventions to address pollution or ecological imbalances. Acid rain and industrial wastewater discharge can lead to water acidification, affecting ecosystems and human health. By regulating and controlling water pH, the impact of external factors such as acid rain can be mitigated, helping to protect the environment.
How to Measure Water pH?
pH Monitoring for Pools and Household Water
1. pH test strips
This is the simplest and fastest method, suitable for rough estimation of water’s acidity or alkalinity. To use, immerse the strip into the water sample and compare the color change with a reference color chart to estimate the pH value. This method is easy to use and cost-effective, making it suitable for household or initial testing, but it has lower accuracy.
2. Portable pH meters
This is an electronic device that, after calibration, is inserted into the water. Once the reading stabilizes, the pH value can be taken. It provides a more accurate pH measurement. Portable pH meters are suitable for household water, drinking water monitoring, and daily pool water quality checks. Calibration (usually using standard buffer solutions) is required before use to ensure measurement accuracy.
pH Monitoring for Aquaculture and Rivers
1. Online monitoring systems
Online water quality monitoring consists of water quality sensors, data acquisition hosts, and management platforms, forming an automated monitoring system. These systems integrate the water pH sensor, data logging, transmission, and alarm function, allowing for real-time continuous monitoring of water quality changes.
Online water pH monitoring systems use high-precision pH sensors as measurement units. The weak analog signals collected by the sensor are amplified, filtered, and temperature-compensated before being converted into digital signals and uploaded to the management platform. This allows for continuous monitoring of pH changes in the water. The system has data analysis and exceedance alarm functions, significantly improving water quality management efficiency.
However, compared to other measurement methods, online water pH monitoring systems are more expensive and require regular maintenance. This is because pH sensors are susceptible to interference from suspended particles in the water, necessitating periodic maintenance and calibration. These factors should be considered when using the system.
2. Portable water quality analyzer
A pH water quality analyzer is a portable testing device based on the glass electrode method. Its working principle relies on a composite probe consisting of a glass electrode and a reference electrode to detect the potential difference generated by the hydrogen ion concentration in water. Combined with a built-in temperature sensor, it performs automatic temperature compensation (ATC) and converts the electrical signal into a displayed pH value.
The portable water quality analyzer is compact, easy to carry, and has a fast response time. It can measure both water pH and temperature values and features data storage and over-limit alarm functions. It is widely used in aquaculture, environmental monitoring, and field investigations. Similar to online pH monitoring systems, its pH electrode also requires regular maintenance and calibration.
How to Decrease pH of Water?
Lowering the water pH(acidification treatment) usually requires adding an appropriate amount of acidic substances to increase the acidity of the water, thereby achieving the purpose of reducing the pH value. The appropriate method should be selected based on the application scenario, with common methods including the addition of acidic chemical agents and the injection of carbon dioxide. Acidic chemical agents include the strong and fast-acting hydrochloric acid, the highly corrosive sulfuric acid, and the safer and milder acetic acid/citric acid. Specific recommended solutions are as follows:
| Methods for lowering water pH in different scenarios | ||
|---|---|---|
| Scenario | Recommended method | Expected effect |
| Home fish tank | Citric acid or CO₂ diffusion | Safe and controllable, pH stable at 6.5-7.0 |
| Aquaculture | Aluminum sulfate (alum) or humic acid | Has both flocculation and acidification functions |
| Industrial wastewater treatment | Hydrochloric acid/sulfuric acid + automatic dosing system | Fast and accurate, can be linked to pH online monitoring |
| Drinking water treatment | Food-grade phosphoric acid or CO₂ injection | Meets hygiene standards, no residual risk |
How to Raise pH in Water?
Increasing the water pH(alkalization treatment) is mainly achieved by adding alkaline substances. The most common methods include adding alkaline chemical agents and injecting alkaline gases (or minerals). The specific operation involves diluting an appropriate amount of sodium hydroxide (caustic soda), sodium carbonate (soda ash), or sodium bicarbonate (baking soda) in a predetermined ratio, then slowly adding it to the water in batches while continuously stirring to ensure even distribution. A pH meter should be used to monitor water quality changes in real-time until the desired pH range is reached. The appropriate method should be selected based on the application scenario, and the following are recommended methods for increasing pH in different situations:
| Methods for reducing water pH in different scenarios | ||
|---|---|---|
| Scenario | Recommended method | Expected effect |
| Family fish tank | Sodium bicarbonate (baking soda) | Safe and controllable, pH stable at 7.5-8.0 |
| Aquaculture | Quicklime or shell sand as bottom | Increasing pH and increasing calcium ions |
| Industrial wastewater treatment | Sodium hydroxide + automatic dosing system | Fast and accurate, can be linked to online monitoring |
| Drinking water treatment | Food grade sodium carbonate (soda) | Meet hygiene standards, no residual risk |